Enabling Continuous Improvement in Food Safety
Identifying non-conformities is only the first step in food safety management. The real value lies in how these issues are analyzed, addressed, and prevented from recurring. In many organizations, CAPA processes are still managed through fragmented records and manual follow-ups. This often leads to repeated issues and inconsistencies during audits.
Digital CAPA management transforms corrective actions into a structured and traceable process. This approach strengthens both operational control and audit readiness.

What Is CAPA and Why Is It Critical in FSMS?
Corrective and Preventive Actions (CAPA) are structured processes designed to
• identify non-conformities
• analyze root causes
• implement effective actions
• prevent recurrence
Without an effective CAPA system, data from EMP, sanitation, and IPM activities remain isolated. As a result, this data fails to translate into meaningful operational improvement.
Limitations of Traditional CAPA Management
Manual or semi-digital CAPA processes commonly lead to
• incomplete follow-up
• unclear accountability
• weak root cause analysis
• difficulties in retrieving historical records during audits
As a result, CAPA becomes a reactive and compliance-focused task, rather than a proactive improvement tool.
What Is Digital CAPA Management?
Digital CAPA management centralizes the entire lifecycle of corrective and preventive actions—from identification to closure—within a single system.
This approach
• standardizes workflows
• ensures traceability
• links actions to evidence
• enables performance measurement
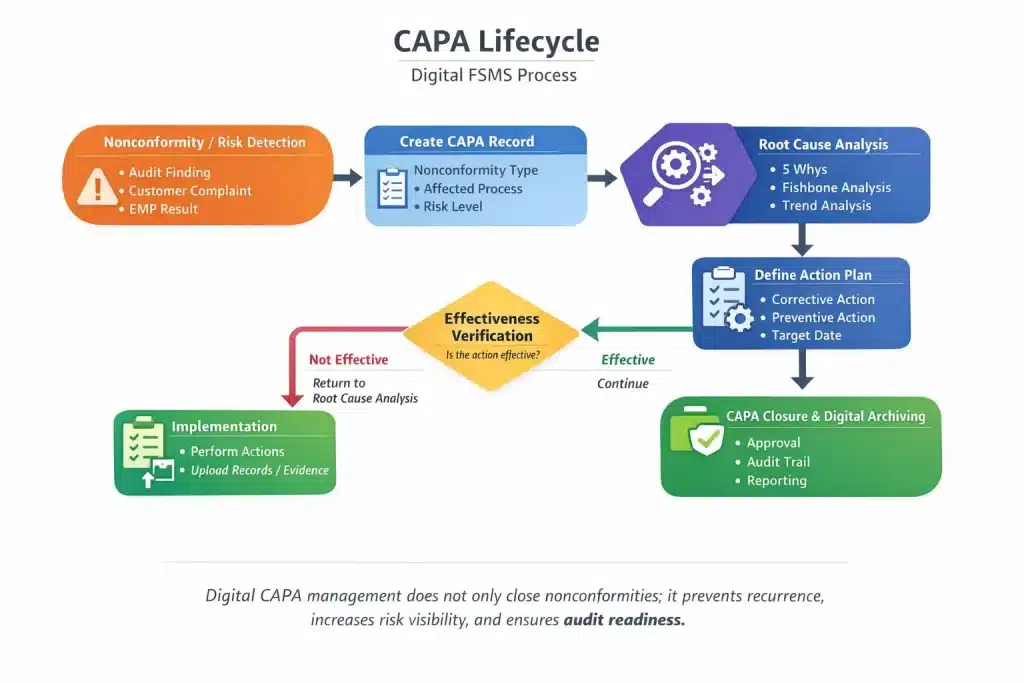
Key Stages of a Digital CAPA Workflow
Table: Digital CAPA Lifecycle
| Stage | Description |
|---|---|
| Non-Conformity Identification | EMP, sanitation, IPM, or audit findings |
| Root Cause Analysis | 5 Whys, Fishbone, etc |
| Action Definition | Corrective or preventive action |
| Responsibility & Deadline | Assigned ownership and timelines |
| Verification | Effectiveness check |
| Closure | Audit-ready documentation |
Sample Visual
Integrating CAPA with EMP, Sanitation, and IPM
Within a digital FSMS
• EMP positive results can automatically trigger CAPA
• sanitation performance validates corrective actions
• IPM data supports environmental risk assessment
This integration accelerates root cause analysis and reduces recurring non-conpliances.
Business Benefits of Digital CAPA Management
• Improved audit performance
• Faster response to non-conformities
• Reduced recurrence of issues
• Clear accountability
• Stronger continuous improvement culture
Making CAPA a Core Element of Food Safety Excellence
Digital CAPA management elevates corrective and preventive actions from isolated records to a core operational process within FSMS.
By integrating CAPA with EMP, sanitation, and IPM modules, food manufacturers achieve
• better visibility
• stronger control
• sustainable food safety performance
Long-term food safety success does not depend solely on identifying issues. It depends on eliminating them permanently through structured and continuous improvement.
Next Steps
For food companies seeking efficiency, Qualiqo offers a reliable, all-in-one sanitation management solution. Qualiqo is designed to streamline food safety and sanitation processes for better operational control. It helps businesses track cleaning schedules, verify tasks, and meet food safety standards. Features include audit management, real-time alerts, and complete traceability across operations. With Qualiqo, food businesses embrace digital transformation and reinforce their food safety commitment.
Did you get enough information about “Digital Management of Corrective and Preventive Actions (CAPA) in FSMS“
Qualiqo is here to help you. It answers your questions about sanitation and hygiene, Lab. & EMP, IPM and Pest Control. We also provide information about the main features and benefits of the software.
We help you access the Qualiqo demo and even get a free trial.